Fill in application form online

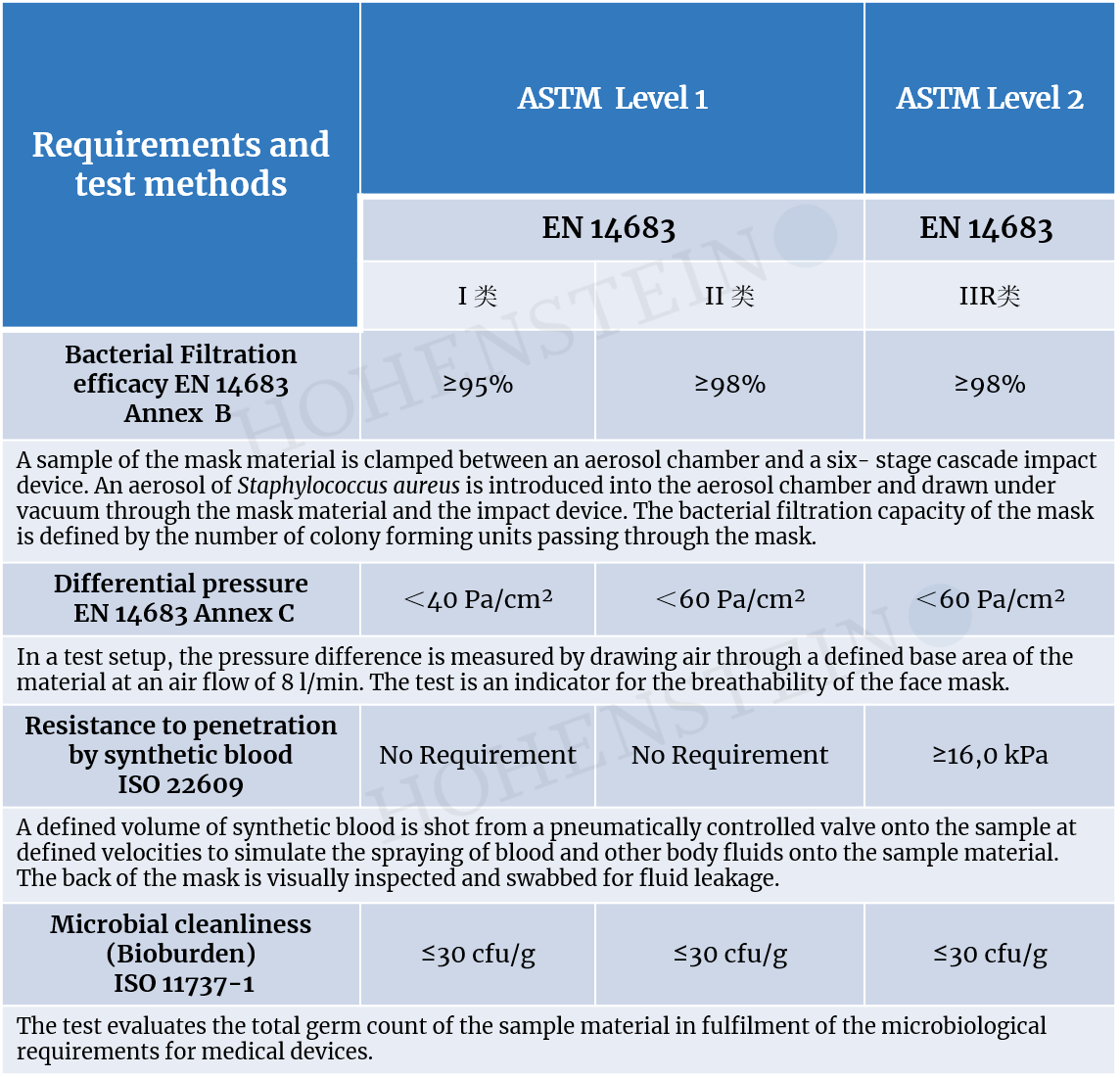
Microbiological-hygienic + physical testing of face masks.
As a testing laboratory for medical products, we test the microbiological-hygienic and physical functionality of medical face masks. We test masks (for the mouth and nose) that are usually worn by doctors and care staff to protect patients or residents of care homes from pathogens spread by the medical staff. These “surgical masks” or “oronasal masks,” can provide some protection for medical staff from splashes during surgical procedures.Medical face masks can also play an important role in the containment of epidemic or pandemic situations by reducing the spread of infections.
Protecting the wearer from smear infections caused by accidental contact of their contaminated hands with their nose and mouth.
Protecting the environment around infected persons from droplet contamination caused by pathogens ejected during coughing and sneezing.
Cytotoxicity test.
The test on cytotoxicity according to DIN EN ISO 10993-5 is the basis of numerous tests for biocompatibility under the standard series DIN EN ISO 10993 and is accredited by DAkkS at the Hohenstein Laboratories. In this cell culture test skin cells are used to detect cell damaging substances that may leach out of the sample material. Therefore, the test allows the evaluation of the potential for cell damage. This potential is recorded as a summation parameter.For this purpose an exctract of the test material is prepared, which is cultivated with L 929 skin cells for several days. The cell viability respectively potential cell-toxic effect is quantitatively determined for the treated cell culture in comparison with untreated control cultures. A growth inhibition of more than 30 % in comparison with the extracting agent control is assessed as a clear cell-toxic effect.
Q & A
-
What services can Hohenstein provide for medical face masks?
Hohenstein tests the microbiological-hygienic and physical functionality of medical face masks (not N95 masks). We can also test the material quality and certify to STANDARD 100 by OEKO-TEX®.We support producers in providing technical documentation as proof of effectiveness and safety, a requirement for medical product approval in accordance with the EU Medical Device Regulation 2017/745.Hohenstein does not provide the additional tests and documents required for final approval as a medical device.
-
Does Hohenstein test respiratory/FFP/N95 masks?
No, we do not currently test filtering face pieces (FFP). FFP masks protect the wearer from breathing in possible pathogens from aerosols or from solid or liquid particles.
-
Does also Hohenstein test non-medical oronasal/community masks?
Yes, Hohenstein offers standard tests for oronasal masks / medical face masks that cover valid legal requirements.These masks are NOT considered protective masks. However, we are happy to support manufacturers in checking the functionality of non-medical masks to ensure that consumers get the best possible product.
-
Flyer_Protective Masks Testing
-
Factsheet_Medical Face Masks